1910-1999

Rayner is Founded (1910)
In 1910, JB Reiner and CD Keeler founded the Rayner Company in London, England. One of the early customers for his first spectacles was a four-year old boy named Harold Ridley.
This association with Rayner continued into Mr. Ridley's professional career. Ridley worked with Rayner's optical scientist John Pike on the first televised eye operations (black and white in 1948 and colour in 1950) and a system for examining the inner eye using electronic rather than optical methods (1949).
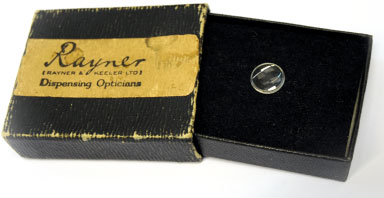
Harold Ridley and the Birth of the IOL (1949)
Ridley, together with John Pike of Rayner and John Holt of ICI, designed the first IOL. Rayner manufactured this first lens at its instrument workshop in Brighton, East Sussex. The first IOL operation was performed on a 45 year old woman at St Thomas's Hospital in London on 29 November, 1949.
Download Harold Ridley and the Invention of the IOL>
Ridley Presents IOL to Oxford Congress (1951)
Ridley presented his first paper on IOLs at the Oxford Congress in England on 9 July. The reaction from his professional colleagues was hostile. Ridley's results were also met with disdain and scepticism at a meeting of the AAO in Chicago on 12 October 1952.

Choyce MkI (1956)
Rayner introduced the Choyce MkI, Peter Choyce's modification of Strampelli's anterior chamber lens design.
Surgeons Approach Rayner With Suggestions for Lens Designs (1952-58)
Rayner began work with Strampelli, Epstein, Joaquin Barraquer and Dannheim on their designs for posterior and anterior chamber lenses.

Binkhorst Four-Loop Design (1962)
Work began with Binkhorst on his four-loop iris fixation design. Fyodorov's modification of Binkhorst's lens was also introduced during this decade. In 1965, Binkhorst's two-loop design demonstrated the advantages of iridocapsular fixation following planned extracapsular cataract extraction (ECCE).


The Intraocular Implant Club is Formed (1966)
The pioneering surgeons formed the Intraocular Implant Club (IIC) with Ridley as their first President and John Pike of Rayner as a founder member. In 1975 this became the International Intraocular Implant Club (IIIC) as the work of the pioneers became recognised worldwide.
L-R Back: John Pike of Rayner (UK), Murto (USA), Michael Roper-Hall (UK), Svyatoslav Fyodorov (USSR), Neil Dallas (UK), C.A. Brown, Rubenstein, Warren Reese (USA),Leonard Lurie (UK).
L-R Front: Jørn Boberg-Ans (Denmark), Cornelius Binkhorst (Holland), Peter Choyce (UK), Harold Ridley (UK), Benedetto Strampelli (Italy), Dr Edward Epstein (South Africa), MMe Boberg-Ans (Denmark).

Modern Posterior Chamber IOLs (1975)
The first, modern, posterior chamber lens design was developed with John L. Pearce. A joint venture and technology transfer agreement was made between Rayner and Coburn, USA permitting the Americans to manufacture IOLs.
First FDA Approved Lenses (1979-1981)

The Choyce MkVIII and MkIX anterior chamber designs became the first lenses to be approved by the FDA as safe and effective. Around this time, Rayner engraved the dioptric power of lenses onto the optic surface.
All Rayner Products Have CE Mark Granted (1986-1999)
- Rayner move to a new custom-built manufacturing laboratory in Hove, East Sussex.
- All Rayner manufactured products receive CE Mark approval.
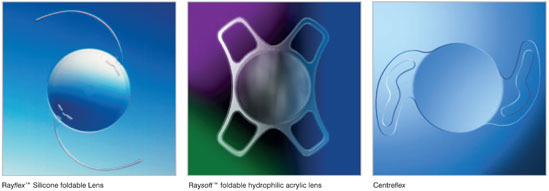
- Rayner launch the Rayflex silicone foldable lens and Rayvisc , a sodium hyaluronate viscoelastic.
- Rayner introduce the Raysoft foldable hydrophilic acrylic lens.
- The proprietary Centerflex® (570H) design, an inventive Rayner lens featuring the unique Anti-Vaulting-Haptic technology, was launched in 1999. This would prove to be a major milestone for the company (see 2003)
- Rayner celebrate 50 years of implant design and manufacture.

AVH Technology®
AVH (Anti-Vaulting Haptic) Technology is unique to Rayner IOLs and offers superior centration and stability within the capsular bag leading to accurate, predictable and sustainable refractive outcomes.
2000 -2010
Enhanced Square Edge
The Amon-Apple Enhanced Square Edge is another unique Rayner innovation and was designed in collaboration with the late Professor David J. Apple (Salt Lake City, USA) and Professor Michael Amon (Vienna, Austria).
Only Rayner injectable IOLs feature the Amon-Apple Enhanced Square Edge which creates a continuous physical barrier to lenticular epithelial cell migration, the pre-cursor to posterior capsular opacification (PCO).
Rayner IOLs with the Amon-Apple Enhanced Square Edge are noted for exceptionally low rates of PCO.

C-flex Spherical Monofocal IOL (2003)
C-flex was the first Rayner IOL to incorporate both AVH Technology and the Amon-Apple Enhanced Square Edge. Based on the original and very successful Centerflex design, which was introduced in 1999, C-flex remains one of the most implanted IOLs worldwide.
C-flex was the first IOL from a non-American IOL manufacturer in over two decades to gain FDA approval (May 2007).

.jpg)
Superflex (2003)
Superflex was introduced to give all the advantages of C-flex but with a larger optic (6.25mm) and a longer overall length (12.50mm).In addition to being anatomically suited to the larger myopic eye,the larger optic, coupled with the low silicone oil adherence of the Rayacryl material used in all Rayner IOLs, makes Superflex the ideal IOL for the vitreoretinal specialist.
M-flex (2005)
Rayner M-flex Multifocal IOLs represent the latest generation of refractive multifocal IOLs and are indicated for the correction of presbyopia following cataract surgery or PRELEX. Based on Rayner's unique multi-zoned refractive aspheric optic technology, M-flex Multifocal IOLs are associated with exceptional visual outcomes.
M-flex was first implanted on 31st August 2005 by Charles Claoué (London, UK).

T-flex (2005)
The first Rayner injectable toric IOL was implanted by Gerd Auffarth (Heidelberg, Germany) on 4th April 2003. This lens was subsequently modified to become the Rayner T-flex Toric IOL, designed to offer a precise, superior alternative to incisional methods for the treatment of pre-exisiting corneal astigmatism.
With an exceptionally wide range of sphere and cylinder combinations, T-flex can also address the clinical needs of patients with severe astigmatism or patients with a history of corneal pathology.

Sulcoflex Aspheric (2007)
With advanced IOL design and modern surgical techniques, an exact refractive result following cataract surgery with implantation of an IOL is a reasonable expectation. However, even the most careful biometry and surgery can result in a less than optimal patient outcome. Rayner Sulcoflex Pseudophakic Supplementary IOLs, invented by Professor Michael Amon (Vienna, Austria), are indicated for the correction of any post-operative residual refractive errors, without the trauma, or increased surgical risk, of an IOL exchange.

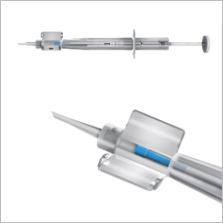
Single Use Soft-Tipped Injector (2006)
The Rayner Single Use Soft-Tipped Injector is designed for the easy and controlled implantation of IOLs within the capsular bag.
Individually supplied with every Rayner IOL (as a "System Pack") the Single Use Soft-Tipped Injector offers a safe and cost-effective method for implanting all Rayner hydrophilic IOLs.
C-flex Spherical Monofocal in the USA (2007)
C-flex was the first IOL from a non-American IOL manufacturer in over two decades to gain FDA approval (May 2007).


C-flex & Superflex Aspheric (2007)
The Rayner C-flex Aspheric and Superflex Aspheric IOLs both incorporate Rayner’s aberration neutral optic technology, offering optimal visual quality and functioning visual acuity in all lighting conditions.

Queen's Award (2009)
In 2009, Rayner was awarded the prestigious Queen's Award for Enterprise, which recognises sustained international trade in overseas markets and growing commercial success. This award placed the company among the most successful businesses in the United Kingdom.
2010 Onwards
M-flex T (2007)
Standard multifocal IOLs are often associated with sub-optimal results in patients with significant corneal astigmatism. With Rayner M-flex T Multifocal Toric IOLs, the combination of the multifocal and toric optical components of T-flex and M-flex allows the benefit of reduced spectacle dependence to be extended to those patients.
M-flex T, the world's first multifocal toric IOL, was implanted by Gerd Auffarth (Heidelberg, Germany) on 28th June 2006.


T-flex Aspheric (2009)
In 2009, the original T-flex IOL (2005) underwent a design update with aspheric technology added to the optical surface, and the T-flex Aspheric toric IOL was launched.
Rayner Introduces RaySert Small Incision IOL Injector System
Designed for use with the Rayner's C-flex range of IOLs (570C and 970C), RaySert assures ease of use with controlled delivery through a convenient single-handed injection system. With RaySert surgeons can perform cataract surgery using wound assisted technique through a small surgical incision, enhancing patient comfort and recovery.
RaySert was launched on April 11 2010 at the ASCRS Symposium on Cataract, IOL, and Refractive Surgery in Boston.

C-flex Advance Aspheric Mini Incision Preloaded IOL Injection System
The C-flex Advance Aspheric combines all the clinically proven benefits of the aberration-neutral C-flex Aspheric IOL, with a mini incision and a preloaded IOL delivery system that eliminates handling of the acrylic lens in Theatre. It also helps reduce the chances of IOL damage or contamination and allows for a Surgically Induced Astigmatism neutral implantation through an incision size of 2.2mm or 2.4mm into the bag. C-flex Advance Aspheric is available in power range +8.0 D to +34.0 D in 0.5 D increments.
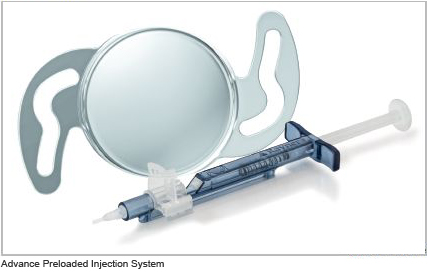
C-flex Aspheric 970C in the USA (2011)
Rayner's C-flex Aspheric 970C with aberration neutral optic technology gains FDA approval and is now available in the US market, in addition to Rayner's spherical monofocal IOL the 570C (see 2007).

RaySert PLUS (2014)
RaySert PLUS is Rayner's newest single use injector, and delivers the C-flex Aspheric or Superflex Aspheric through a 2.2mm mini incision.
The first implant using the new RaySert PLUS injector was successfully delivered through a 2.2mm incision by Allon Barsam in the UK on the 14th March, 2014.

Rayner Complete Acquisition of its Exclusive Distributor in Germany and Austria (2014)
In March, 2014 Rayner completed a significant step forward in its strategic approach to building a greater European market presence.
RaySert PLUS in the USA (2014)
Rayner's RaySert PLUS mini incision injector was launched into the US market at the 2014 AAO in Chicago following its clearance for sale by the FDA.
RaySert® PLUS allows US surgeons to deliver the C-flex® Aspheric IOL (970C) through a 2.2mm clear corneal wound assisted incision.
Rayner Changes the Game at the 2014 ESCRS in London
With the 2014 ESCRS Congress being held in Rayner's home country it was the perfect opportunity to do something special. Having manufactured the world's first IOL in 1949, Rayner decided to mark this event by sponsoring (and curating) the Birth of the IOL Museum, which was housed in an original 1950's London Routemaster bus. Read More

Rayner Surgical Group Limited Acquires Cataract Viscoelastic Portfolio

In July 2015, Rayner acquired the following viscoelastic device products from Aptissen SA (Geneva, Switzerland) as well as assets in their R&D portfolio:
- Ophteis+ 1.4% (Highly Cohesive Sodium Hyaluronate)
- Ophteis 1.0% (Cohesive Sodium Hyaluronate)
- OphteisMax 2.5% (Visco-Adaptive Sodium Hyaluronate)
- OphteisBio 1.6% (Cohesive-Dispersive Sodium Hyaluronate)
- OphteisBio 1.8% (Cohesive-Dispersive+ Sodium Hyaluronate)
- OphteisBio 3.0% (Dispersive Sodium Hyaluronate)
- Methylvisc 2.0% (HPMC)
Rayner Defines the New Standard in Ophthalmic Visco-Surgical Devices (OVD) with Ophteis FR Pro (2016)

On March 7th 2016, Rayner launched a new OVD – Ophteis® FR Pro. Ophteis® FR Pro with sorbitol is a revolution in OVDs, protecting the corneal endothelium from free radical energy caused by phaco emulsification.
Aside from its unique free radical scavenging qualities, Ophteis® FR Pro does everything a surgeon demands from an OVD and more. The addition of sorbitol enables the OVD to perform as a visco-cohesive, providing excellent all-round performance at every stage of surgery. It is stable in the anterior chamber during phaco, providing superior chamber maintenance. What’s more, it doesn’t require refrigeration, so it’s ready to go when you are.
The Grand Opening of The Ridley Innovation Centre (2016)

In 2016, Rayner transferred its global headquarters to The Ridley Innovation Centre, our new £20million R&D, training and production facility in Worthing, West Sussex. This state of the art, dedicated intraocular lens (IOL) production facility has the capability of manufacturing 3 million intraocular lenses each year.
The new centre was also designed with the surgeon at its heart to enable training, education and research. Featuring a wet lab, library, training facilities and even a museum on the history of the IOL, this enables Rayner to engage and partner surgeons and academics in new product development
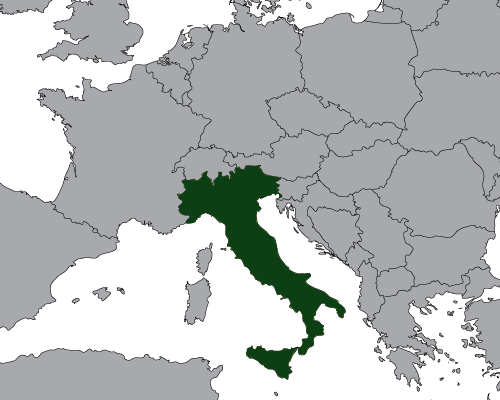
Rayner Continues Growth in Europe with Direct Presence in Italy (2016)
On the 6th July 2016, Rayner announced its direct entry into the Italian cataract market.
The entire Rayner IOL and injector portfolio, available in Italy for the last 20 years via independent distributors is now available through Rayner Italia Srl.
Simonetta Morselli MD, San Bassano Hospital, Italy, commented “We are very pleased that Rayner has entered the Italian market. Rayner has a strong heritage in developing the world’s first IOL, and we are excited about the opportunity to work directly with them”.
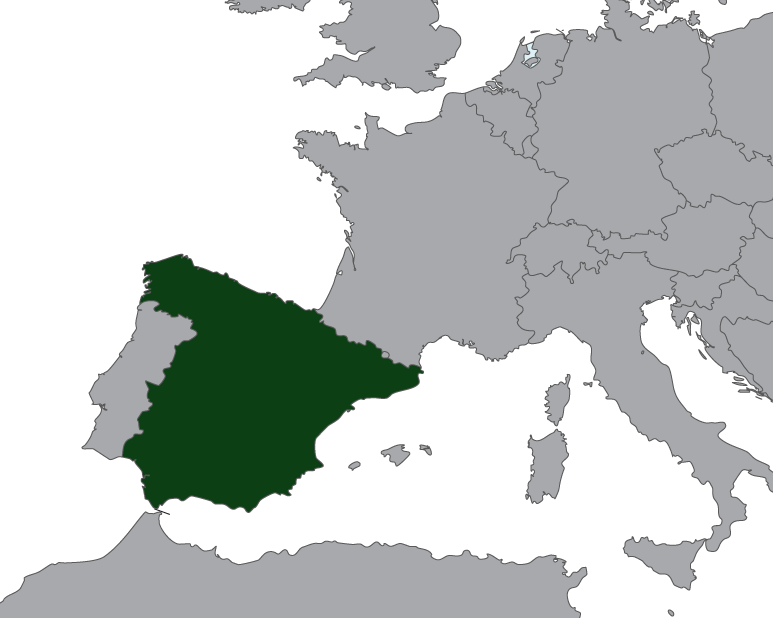
Rayner Announces Direct Entry Into Spain (2016)
The entire Rayner IOL and injector portfolio, available in Spain for the last 20 years via our independent distributor Rayner Iberica SA., is now available through our wholly owned subsidiary, Rayner Surgical S.L.
Spain’s strategic importance
Not only is Spain the 5th largest cataract market in Europe (by volume), it also has the highest percentage conversion to premium IOLs worldwide. It is strategically important that we strengthen our relationships and work more closely with surgeons in such highly refractive markets, as we build towards the launch of our trifocal technology.
“I would like to warmly welcome Rayner to Spain. With closer access to their latest technology, I am confident there will be an improvement of intraocular lenses, which will ultimately translate to greater patient satisfaction” said Elena Barraquer, Medical Director Barraquer Clinic, commenting on the achievement.
.jpg)
The Smallest Fully Preloaded IOL Incision: RayOne (2016)
Following the receipt of CE mark approval, Rayner announced the launch of RayOne®: our fully preloaded IOL injection system, unveiled at the 2016 ESCRS in Copenhagen.
RayOne® offers the following features and benefits:
- Lens and injector designed as one to enable the smallest fully preloaded IOL incision
- Enhanced 6.0 mm optic, retaining proven stability and optical performance of existing platform
- One system for all patients with the largest fully preloaded power range (-10 to +34 D)
RayOne® offers an injector which delivers the IOL consistently, with expert control, through a micro incision with minimal wound stretch, including patented Lock & Roll™ technology for the smallest fully preloaded IOL incision.
Rayner Acquires Moorfields Pharmaceuticals (2016)

On 3rd October 2016, Rayner announced that it had acquired the business and assets of Moorfields Pharmaceuticals from Moorfields Eye Hospital NHS Foundation Trust for the purpose of launching a specialist ophthalmic pharmaceuticals division that will be expanded across Rayner’s direct presence in Europe.
The acquisition closed 30th September 2016.
Commenting on the acquisition, Rayner CEO, Tim Clover, remarked, “We are committed to supporting ophthalmologists in attaining the best outcomes for their patients. Although the intraocular lens plays a leading role in the visual outcomes of cataract and refractive surgery, the pharmaceuticals that manage the tear film and inflammation are critical to the end result. For that reason, the acquisition of Moorfields Pharmaceuticals will enable us to offer an even wider range of ophthalmic tools that aid and streamline the surgical process whilst remaining focused on the visual outcome for the patient. We are delighted to welcome Moorfields Pharmaceuticals to the Rayner team.”
Rayner Announces Direct Presence in Portugal (2017)
Following on from their European expansion in 2016 -- direct entry into the Italian cataract market and direct entry into the Spanish cataract market -- Rayner announced its direct entry into the Portuguese cataract market.
Based out of Porto, the entire Rayner IOL & injector portfolio, previously available through a network of distributors, will now be available through Rayner Surgical S.L. (Portugal).
Phoenix Equity Partners announces investment in Rayner Surgical Group (2017)
Phoenix Equity Partners ("Phoenix"), a leading UK middle-market private equity firm, today announces an investment in Rayner Surgical Group (“Rayner” or “the Company”) alongside the current management team, led by CEO Tim Clover, and a number of the Company’s existing shareholders. The deal is Phoenix’s second from its new 2016 Fund and will provide Rayner with additional investment to spur the Company’s continued growth.
Tim Clover, CEO of Rayner, commented: “In the past two years we have focussed on strengthening R&D and external collaborations with surgeons and universities. This has led to an exciting new product pipeline placing Rayner at the forefront of innovation in ophthalmology. Our partnership with Phoenix gives us the support and resources to develop and launch these technologies, contributing to the improvements in visual outcomes for surgeons and patients worldwide.”
Rayner Strengthens Commitment to US Market With Subsidiary Rayner Surgical Inc. (2017)

Rayner announced its investment into the US market with the establishment of a dedicated subsidiary, ‘Rayner Surgical Inc.’ Based out of New York, Rayner Surgical Inc. will provide US specific marketing, regulatory, commercial and research functions to Rayner. From its offices at 100 Park Avenue, NYC, Rayner Surgical Inc. will actively promote and support the growth and development of Rayner’s business in the US.
What Does This Mean for US Surgeons?
A new, physical US presence will strengthen Rayner’s relationships with US surgeons, universities and key opinion leaders (KOLs), generating valuable collaborative partnerships and identifying new opportunities in IOL design and innovation. Where clinical studies in the US are required, Rayner Surgical Inc. will lead in identifying suitable providers, investigators and investigation sites and in study design and management. The highest levels of customer service and greater knowledge of customer requirements is expected to lead to an exciting new IOL product pipeline for the American market, contributing to improvements in visual outcomes for clinicians and patients.
New Patented Trifocal Technology. Rayner Launch RayOne® Trifocal at the ESCRS (2017)

Rayner launched the newest member of the RayOne® family of preloaded IOLs: the RayOne® Trifocal, to be unveiled at the upcoming 2017 ESCRS conference in Lisbon.
The most advanced lens design Rayner has ever produced.
Based on the proven, high performance Rayner ‘closed loop’ platform, the new diffractive technology of the RayOne® Trifocal is the result of several years of development in partnership with a leading European Research Centre using advanced vision simulation technology. The resultant new patented diffractive trifocal profile reduces light loss to an industry leading 11% and provides a smooth transition for the patient from near to intermediate and distance vision.
